Understanding Direct Evaporative Cooling
To understand how an evaporative cooler works, it is necessary to understand something about the properties of heat, air and water vapour. The most common type of Evaporative Cooler is the Direct type, in which the hot outside air is cooled within the machine and forced into the building and exhausted to outside again. Other types are Indirect and Air Washer. In this article, we will only focus on Direct Evaporative Cooling.
What is heat?
Before we can discuss the cooling process we must understand a little of the nature of heat which exists in two forms: Sensible heat (that you can feel or “sense”) and Latent heat (hidden heat that cannot be detected with a thermometer).
The heat used to evaporate water into water vapour is called “Latent Heat of Evaporation”. For example, it is the heat from the hot pavement that is given up to evaporate the water after a summer rainstorm, or the heat from the stove burner given up to evaporate the water in a boiling kettle. As the liquid water changes its state into vapour, (you can’t see water vapour) it absorbs heat from its surroundings; the temperature does not change but the amount of heat or energy it absorbs is contained in the molecular structure of the vapour. Evaporative Cooling is only possible because of this natural phenomenon of Latent Heat.
Where does Latent Heat come from?
It comes from surrounding air and materials. Whenever a substance changes its state from solid to liquid (ice to water) and from liquid to vapour (water to vapour or water to steam), it absorbs heat from the surroundings. That means that the surrounding air and solid objects and liquids become cooler as they yield up their heat to the melting or evaporating process.
Total Heat is the sum of latent heat and sensible heat. It is the total amount of heat in a room, made up of heat you can feel and heat you can’t feel. Total heat is measured in kilojoules (kJ):1000kJ is approximately 1000 BTU’s. The complete evaporation of one litre of water absorbs about 2000kJ of heat energy and that occurs within the process without any external energy input. That is why evaporative air conditioners use a very small amount of electrical power to operate. The only power that is required is for driving the fan and pump.
The Evaporative Air Cooling process
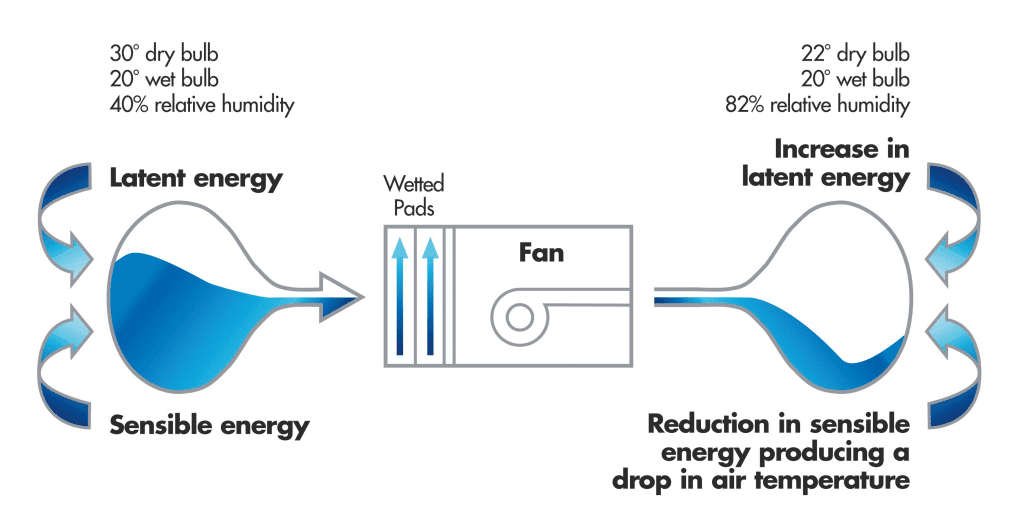
 In Direct Evaporative Air Cooling machines, the heat exchange process is enabled by means of a water pump that delivers water to cooling pads and a motor driven fan that forces hot outside air through those media panels. These components combine to accelerate the natural heat exchange process.
In Direct Evaporative Air Cooling machines, the heat exchange process is enabled by means of a water pump that delivers water to cooling pads and a motor driven fan that forces hot outside air through those media panels. These components combine to accelerate the natural heat exchange process.
During the process, some of the sensible heat from the air (the heat you can feel) is changed into latent heat (the heat you can’t feel) when water in the Evaporative Air Cooler is changed into water vapour.
This process of sensible heat changing into latent heat causes the hot air to become colder since some of its (sensible) heat has been used as explained above. So the air temperature falls. The cold air is then pumped into the building and is eventually exhausted from the building. It is never re-circulated.
Evaporative Air Coolers will slightly increase the humidity inside the building. However, we need to remember that the temperature has also dropped. It is the combination of temperature  and humidity that creates human comfort, and Evaporative Air Coolers are used so widely around the world because they can create comfortable conditions. For example, 80% humidity and 30°C is very uncomfortable, but 80% humidity and 16°C is quite comfortable. Furthermore, comfort is also improved by increasing air velocity in hot conditions and Evaporative Air Coolers create sufficient air movement to also minimise the effects of humidity.
and humidity that creates human comfort, and Evaporative Air Coolers are used so widely around the world because they can create comfortable conditions. For example, 80% humidity and 30°C is very uncomfortable, but 80% humidity and 16°C is quite comfortable. Furthermore, comfort is also improved by increasing air velocity in hot conditions and Evaporative Air Coolers create sufficient air movement to also minimise the effects of humidity.
As we can see from the below graph, temperature and humidity are inversely proportional: during the portion of the day when temperatures are higher, relative humidity is lower. This is why Evaporative Cooling technologies are effective, they work better when temperature is high, because a lower relative humidity leaves space for evaporation to occur.
To understand how an Evaporative Cooler such Breezair works, it is necessary to have a clear idea of Psychrometrics: this consists of the interactions between heat, moisture and air, and specifically how to change air from one condition to another. As temperature rises, its capacity to hold moisture rises as well: moisture is therefore a very influential factor for heat gain. The interaction between the three elements stated above can be explained in a single chart, called a Psychrometric Chart.
Let’s clarify the elements of the chart itself:
- DRY BULB TEMPERATURE (DBT) Refers to the ambient air temperature measured using a normal thermometer: it is called “dry” because it is not affected by the moisture of the air. The dry bulb temperature is an indicator of heat content of the air if all other factors remain constant. As the DBT temperature increases, the capacity of moisture the airspace will hold also increases.
- WET BULB TEMPERATURE (WBT): The Wet Bulb temperature is the temperature measured by using a thermometer whose glass bulb is covered by a wet cloth. The wet bulb temperature indicates the moisture content of air. It is extremely important to consider the WBT for evaporating cooling processes because the difference between the dry bulb and wet bulb temperature is a measure of the cooling efficiency. At 100% relative humidity, the wet bulb temperature equals dry bulb temperature.
- HUMIDITY: Describes the quantity of water vapour in the air. We can consider two types of humidity:
- Absolute humidity: the mass of water vapour present in a given mass of air, expressed in grams of water vapour (g) per kilogram of dry air (kg).
- Relative humidity (RH): the actual amount of moisture in the air compared to the total or maximum moisture the air can hold at a given temperature. When air has 50 percent RH, we say it is 50 percent saturated. As air approaches 100 percent saturation, it can take on less and less water until at 100 percent RH, the air cannot hold more water.
The chart to the right represents a typical summer day showing temperature and relative humidity in non-tropical region.
If we have two of these elements, using the Psychrometric Chart we can easily find the third one: for example, given a certain Dry Bulb Temperature and a certain Wet Bulb Temperature, it is easy to find the Relative Humidity, by checking the intersection of these two values.
In Evaporative cooling, we move in the chart along lines of constant enthalpy: this means we are talking about an adiabatic process, where no transfer of heat occurs.
When humidifying the air with evaporation, the molecules of air take with them a part of water vapour, as well as latent heat, which therefore represents the amount of moisture in the air. Taking a part of the latent heat, the air entering the building after having passed through the unit is then cooler.
Moving in the psychrometric chart from point A along a line of constant enthalpy we are adding moisture to the air (vertical axis), and proportionally obtaining a drop in Dry Bulb Temperature (horizontal axis). With evaporative cooling, the possible maximum reduction in temperature is the differential between the Dry Bulb and the Wet Bulb temperature (called Wet Bulb Depression): this means we could move along the line until point C, where Relative Humidity is 100%. However, no equipment is perfect, so there will be certain losses in the cooler: if we consider a cooler 90% efficient, we can then reach point B, therefore obtaining a temperature drop of 11.5°C.